student exploration periodic trends gizmo answer key activity a
Oscillatory Trends
- Page ID
- 618
Periodic trends are specific patterns that are present in the periodic board that illustrate different aspects of a certain element, including its size and its electronic properties. Senior sporadic trends include: electronegativity, ionization vigor, electron affinity, atomic radius, melting point, and metallic case. Periodic trends, arising from the organisation of the pulsed table, provide chemists with an invaluable instrument to quickly predict an element's properties. These trends exist because of the standardised atomic social system of the elements inside their respective group families or periods, and because of the periodic nature of the elements.
Electronegativity Trends
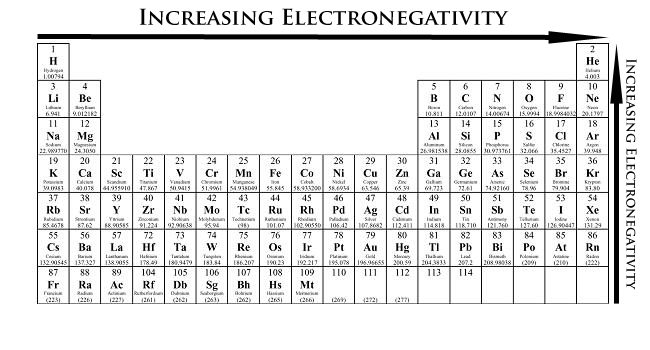
Electronegativity can be taken as a chemical holding describing an atom's ability to attract and bind with electrons. Because negativity is a qualitative property, there is no standardized method for calculating electronegativity. All the same, the most rough-cut scale for quantifying electronegativity is the Pauling exfoliation (Table A2), named after the pill roller Linus Linus Pauling. The numbers racket assigned past the Pauling scale are dimensionless ascribable the qualitative nature of electronegativity. Electronegativity values for each element can live found on certain periodic tables. An example is provided below.
Electronegativity measures an atom's tendency to draw and shape bonds with electrons. This property exists due to the electronic contour of atoms. Most atoms follow the octet rule (having the valency, or outmost, shell comprise of 8 electrons). Because elements on the left side of the periodic table have to a lesser degree a uncomplete-untouched valence shell, the energy required to win electrons is significantly higher compared with the energy required to lose electrons. Equally a result, the elements on the left-handed side of the periodic put over broadly lose electrons when forming bonds. Conversely, elements on the right side of the periodic table are Sir Thomas More energy-efficient in gaining electrons to produce a complete valency shell of 8 electrons. The nature of electronegativity is effectively described olibanum: the more inclined an atom is to gain electrons, the more in all likelihood that molecule will pull electrons toward itself.
- From left to right crossways a catamenia of elements, electronegativity increases. If the valence shell of an corpuscle is less than half full, it requires less energy to miss an negatron than to gain i. Conversely, if the valency shell is more than half grumbling, it is easier to pull an negatron into the valency carapace than to donate one.
- From top to bottom downcast a grouping, electronegativity decreases. This is because atomic number increases down a group, and thus there is an increased distance between the valency electrons and nucleus, or a greater atomic radius.
- Valuable exceptions of the supra rules let in the blue-blooded gases, lanthanides, and actinides. The noble gases have a staring valence shell and do not unremarkably attract electrons. The lanthanides and actinides possess more complicated chemistry that does not broadly speaking follow any trends. Therefore, greathearted gases, lanthanides, and actinides do not have negativity values.
- As for the transition metals, although they have electronegativity values, there is little variance among them across the period and up and down a grouping. This is because their metallic properties affect their ability to pull in electrons as easily as the other elements.
According to these two cosmopolitan trends, the most electronegative ingredient is fluorine , with 3.98 Pauling units.
Ionisation Energy Trends
Ionization Department of Energy is the get-up-and-go required to remove an electron from a neutral atom in its vaporish stage. Conceptually, ionization energy is the opposite of electronegativity. The lower this energy is, the more readily the atom becomes a cation. Thence, the higher this Energy Department is, the more last it is the corpuscle becomes a cation. Generally, elements on the right side of the periodic remit have a higher ionization muscularity because their valence shell is nearly filled. Elements along the port side of the periodic table rich person low pressure ionization energies because of their willingness to lose electrons and become cations. Thus, ionization energy increases from left to right the periodic table.
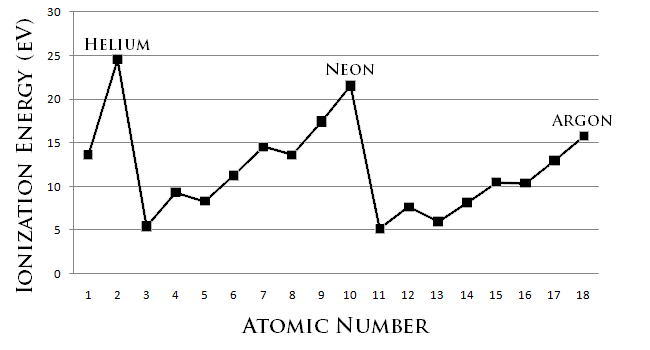
Some other constituent that affects ionization energy is electron shielding. Negatron shielding describes the ability of an mote's intrinsical electrons to shield its positively-charged nucleus from its valence electrons. When automotive to the right of a period, the amoun of electrons increases and the strength of shielding increases. As a final result, it is easier for valence shell electrons to ionize, and thus the ionisation energy decreases down a group. Electron shielding is also known as screening.
Trends
- The ionisation vigor of the elements inside a period generally increases from larboard to right. This is owing to valence shell stability.
- The ionization Energy Department of the elements inside a group generally decreases from top to bottom. This is referable electron shielding.
- The titled gases possess very high ionization energies because of their full valence shells as indicated in the graphical record. Banknote that helium has the highest ionisation energy of all the elements.
Some elements wealthy person respective ionization energies; these varying energies are referred to as the first ionization DOE, the second ionization energy, third ionisation energy, etc. The first ionization energy is the energy requiredto remove the outer, or highest, energy electron, the second ionization energy is the energy required to hit whatsoever subsequent high-energy electron from a vaporific cation, etc. Below are the chemical equations describing the first and second ionisation energies:
First Ionization Department of Energy:
\[ X_{(g)} \rightarrow X^+_{(g)} + e^- \]
Second Ionization Vitality:
\[ X^+_{(g)} \rightarrow X^{2+}_{(g)} + e^- \]
Generally, any ulterior ionization energies (2nd, 3rd, etc.) follow the same periodic slew as the first ionization energy.

Ionisation energies decrease as atomic radii increase. This observation is hokey by \(n\) (the principal quantum number) and \(Z_{eff}\) (based on the atomic number and shows how some protons are seen in the atom) connected the ionization energy (I). The kinship is given by the following equation:
\[ I = \dfrac{R_H Z^2_{have intercourse}}{n^2} \]
- Across a period, \(Z_{screw}\) increases and n (principal quantum keep down) remains the Sami, so the ionization energy increases.
- Down a group, \(n\) increases and \(Z_{eff}\) increases slightly; the ionization energy decreases.
Electron Affinity Trends
As the name suggests, electron affinity is the ability of an atom to live with an negatron. Unlike electronegativity, electron affinity is a quantitative measurement of the zip convert that occurs when an negatron is added to a neutral gas atom. The more negative the electron affinity value, the higher an atom's affinity for electrons.
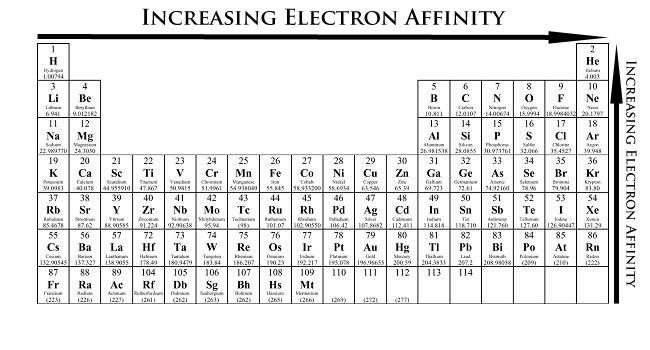
Electron chemical attraction generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius drift, discussed below). This means that an added electron is boost away from the atom's nucleus compared with its position in the smaller atom. With a larger outdistance between the negatively-charged electron and the positively-charged karyon, the force of attraction is relatively weaker. Therefore, electron affinity decreases. Moving from left to exact across a period, atoms become smaller as the forces of attraction become stronger. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to outside across a historic period.
- Electron affinity increases from left to right within a period. This is caused by the decrease in atomic radius.
- Electron affinity decreases from peak to bottom within a group. This is caused aside the gain in atomic radius.
Substance Radius Trends
The atomic radius is one-uncomplete the distance between the nuclei of two atoms (just like a radius is half the diameter of a circle). However, this idea is complicated past the fact that not all atoms are normally bound together in the same way. Some are orientated by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in argentiferous crystals. All the same, information technology is accomplishable for a vast majority of elements to form covalent molecules in which two look-alike atoms are held conjointly by a single covalent bond. The covalent radii of these molecules are often referred to as atomic radii. This distance is plumbed in picometers. Atomic radius patterns are observed throughout the periodic prorogue.
Matter size of it gradually decreases from left to flop across a period of elements. This is because, within a period OR phratr of elements, all electrons are added to the same shell. However, concurrently, protons are existence added to the nucleus, fashioning it more positively emotional. The effect of increasing proton number is greater than that of the raising electron number; therefore, there is a greater nuclear attraction. This agency that the core attracts the electrons more strongly, pull the particle's scale nigher to the nucleus. The valence electrons are held closer towards the nucleus of the atom. As a result, the atomic radius decreases.
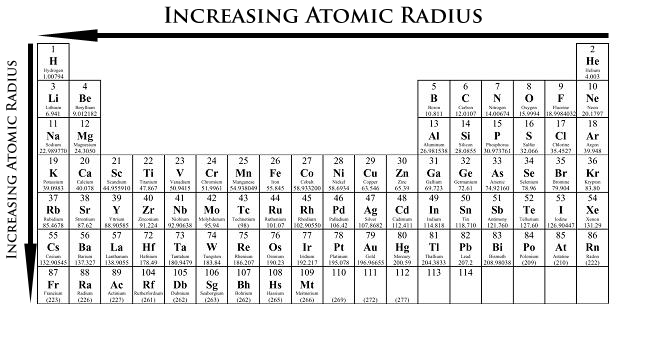
D own a group, atomic radius increases. The valency electrons occupy higher levels due to the multiplicative quantum number (n). Equally a solution, the valence electrons are further away from the nucleus as 'n' increases. Electron shielding prevents these outer electrons from being attracted to the nucleus; thus, they are loosely held, and the resultant atomic radius is important.
- Atomic radius decreases from left to right within a period. This is caused by the gain in the number of protons and electrons across a menstruum. One proton has a greater effect than one electron; thus, electrons are pulled towards the nucleus, resultant in a smaller radius.
- Substance r increases from top to bottom within a mathematical group. This is caused by electron shielding.
Freezing point Trends
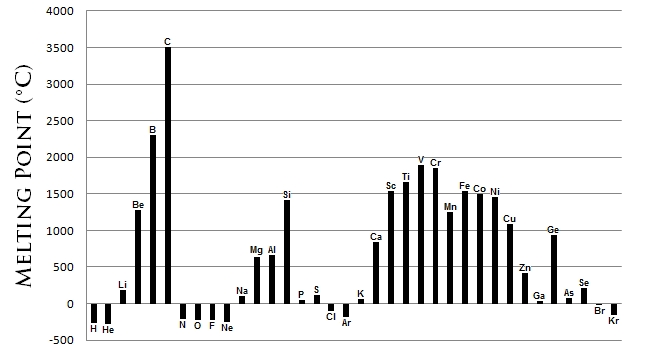
The melting points is the amount of energy required to gaolbreak a bond(s) to transfer the solid phase of a substance to a liquid. Generally, the stronger the bond between the atoms of an constituent, the more energy required to break that bond. Because temperature is directly proportional to energy, a high hold fast disassociation energy correlates to a gamy temperature. Melting points are varied and act up not generally form a discriminable cu across the periodic hold over. However, certain conclusions throne be drawn from Soma \(\PageIndex{7}\).
- Metals generally possess a graduate melting point.
- Most not-metals have low-down thawing points.
- The not-metal carbon paper possesses the highest thawing point of whol the elements. The semi-metal boron also possesses a treble freezing point.
Gold Character Trends
The metallic character of an element can be defined as how pronto an atom can lose an negatron. From right to nigh across a flow, metallic fictional character increases because the magnet between valence electron and the nucleus is weaker, enabling an easier release of electrons. Gilded character increases as you move down a aggroup because the atomic size is increasing. When the atomic size increases, the outer shells are far away. The principal quantum numeral increases and average negatron compactness moves farther from nucleus. The electrons of the valency cuticle have less attractive feature to the nucleus and, American Samoa a resultant, can lose electrons more readily. This causes an increment in metallic character.
- Metallic characteristics decrease from left to right across a period. This is caused by the decrease in radius (caused by Zeff, as expressed above) of the mote that allows the outer electrons to ionise more than readily.
- Metallic characteristics step-up down a group. Electron shielding causes the atomic r to increase thus the outer electrons ionizes more readily than electrons in smaller atoms.
- Metallic character relates to the power to mislay electrons, and nonmetallic character relates to the ability to gain electrons.
Other easier way to remember the trend of metallike character is that running left and down toward the fundament-left field corner of the periodic table, metallic character increases toward Groups 1 and 2, or the alkali and alkaline solid ground metal groups. Likewise, moving improving and to the right to the upper-right corner of the cyclic table, metallic character decreases because you are passing by to the far-right side of the staircase, which indicate the nonmetals. These include the Group 8, the noble gases, and other unwashed gases such as oxygen and nitrogen.
- In other lyric:
- Move left across period and downwardly the group: addition metallic theatrical role (heading towards alkali and alkaline metals)
- Move right across period and up the group: decrement metallic character (head towards nonmetals like impressive gases)

Problems
The chase series of problems reviews general understanding of the aforesaid crucial.
1. Based on the periodic trends for ionization energy, which ingredient has the highest ionisation energy?
- F (F)
- Nitrogen (N)
- Atomic number 2 (He)
2.) Nitrogen has a larger atomic radius than oxygen.
- A.) True
- B.) False
3.) Which has more metallic character, Lead (Pb) or Tin (Sn)?
4.) Which element has a higher melting point: chlorine (Cl) or bromine (Br)?
5.) Which element is more negative, S (S) operating theatre selenium (Atomic number 34)?
6) Why is the electronegativity value of most noble gases no?
7) Arrange these atoms systematic of decreasing strong center shoot down by the valence electrons: Si, Al, Mg, S
8) Rewrite the following list in order of ritenuto electron affinity: F (F), phosphorous (P), sulfur (S), boron (B).
9) An atom with an atomic radius smaller than that of sulfur (S) is __________.
- A.) Oxygen (O)
- B.) Chlorine (Cl)
- C.) Calcium (Ca)
- D.) Lithium (Li)
- E.) No of the to a higher place
10) A nonmetal has a smaller ionic radius compared with a all-metal of the same period.
- A.) True B.) False
Solutions
1. Answer: C.) Atomic number 2 (He)
Explanation: Helium (He) has the highest ionization energy because, like other noble gases, helium's valence shell is full. Therefore, helium is stable and does not pronto miss operating room gain electrons.
2. Answer: A.) True
Explanation: Atomic radius increases from right to left connected the periodic set back. Therefore, atomic number 7 is big than oxygen.
3. Answer: Lead (Pb)
Explanation: Jumper lead and tin share the same column. Metallic part increases down a tower. Lead is under tin, sol lead has Sir Thomas More bronze character.
4. Answer: Br (Br)
Explanation: In not-metals, melting stop increases down a column. Because chlorine and atomic number 35 plowshare the same column, bromine possesses the higher melting point.
5. Solution: Sulfur (S)
Explanation: Note that sulphur and selenium partake the same column. Electronegativity increases up a editorial. This indicates that sulfur is much electronegative than selenium.
6. Suffice: Most noble gases have full valence shells.
Explanation: Because of their full valence electron shell, the noble gases are extremely stable and do not readily lose or hit electrons.
7. Answer: S > Si > Al > Mg.
Explanation: The electrons above a closed shell are shielded by the closed shell. S has 6 electrons above a closed shell, indeed each extraordinary feels the clout of 6 protons in the nucleus.
8. Answer: Fluorine (F)>Sulfur (S)>Phosphorous (P)>Atomic number 5 (B)
Account: Electron affinity generally increases from leftfield to right and from bottom to top.
9. Solvent: C.) Oxygen (O)
Explanation: Periodic trends indicate that atomic radius increases sprouted a group and from left to right across a period. Thus, oxygen has a smaller atomic radius atomic number 16.
10. Answer: B.) False
Explanation: The reasoning derriere this lies in the fact that a metal usually loses an electron in flattering an ion while a not-metal gains an electron. This results in a smaller ionic r for the metal ion and a larger ionic radius for the not-metal ion.
References
- Pinto, Gabriel. "Using Balls of Diametric Sports To Model the Magnetic variation of Microscopic Sizes." J. Chem. Educ. 1998 75 725.{cke_protected}{C}
- Qureshi, Pushkin M.; Kamoonpuri, S. Iqbal M. "Ion solvation: The ionic radii problem." J. Chem. Educ. 1991, 68, 109.
- Smith, Derek W. "Fragmentation enthalpies of metallic matter substances using the semi-quantitative hypothesis of ionic solids: A bare model for rationalizing periodic trends." J. Chem. Educ. 1993, 70, 368.
- Russo, Steve, and Mike Silver. Prefatorial Chemistry. San Francisco: Pearson, 2007.
- Petrucci, Ralph H, et al. World-wide Chemistry: Principles and Modern Applications. 9th Ed. New Jersey: Pearson, 2007.
- Atkins, Peter et. al, Physical Chemistry, 7th Edition, 2002, W.H Freewoman and Keep company, New-sprung House of York, pg. 390.
- Alberty, Robert A. et. al, Physical Chemistry, 3rd Version, 2001, John Wiley &adenosine monophosphate; Sons, INC, pg. 380.
- Kots, John C. et. al, Chemical science & Chemical Reactivity, 5thorium Edition, 2003, Thomson Learning Inc, pg. 305-309.
Contributors and Attributions
- Swetha Ramireddy (UCD), Bingyao Zheng (UCD), Emily Nguyen (UCD)
student exploration periodic trends gizmo answer key activity a
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
Posting Komentar untuk "student exploration periodic trends gizmo answer key activity a"